1 October 2011
Critical for the increasing prevalence of Alzheimer’s disease and mild cognitive impairment are treatments that modify disease progression rather than providing only symptomatic benefit.
Clinical studies commonly utilize a randomized controlled design, where half, of less than half, of the participants receive a placebo for the study duration. This unfortunately denies treatment to many participants, raising ethical concerns and compromising participation [1]. An alternative is the randomized start design (RSD), where participants are randomized to treatment or placebo for an initial period, after which all participants receive the treatment for the remainder of the study. In addition to increasing participation and compliance, Zhang et al. [2] recently hypothesized that RSD has the unique potential to identify disease modifying (DM) effects versus symptomatic effects since the delayed-start group, once switched to treatment, should display a response equivalent to that of the initial treatment group if the treatment addressed only symptomatic effects. However, should the treatment exert DM, the delayed-start group would not be able to catch up to the initial treatment group due to continued disease progression while on placebo. They present a compelling hypothetical description of these phenomena, and data to support or refute this hypothesis is wanting.
In this regard, during our studies of a nutriceutical formulation (NF; folic acid, vitamin B12, vitamin E, S-adenosyl methionine, N-acetyl cysteine, acetyl-L-carnitine) on cognition and mood in Alzheimer’s disease, we included a RSD study of adults with no known or suspected dementia [3-5]. Since inclusion criteria required absence of any known or suspected cognitive impairment, no relevant DM was encompassed within this study. If the hypotheses of Zhang et al. are correct, the delayed-start group should have attained a level of performance equivalent to that of the initial treatment group. Retrospective examination of our findings as presented herein indicate that this indeed was the case, and therefore provide evidence that supports the use of RSD to detect DM versus symptomatic efficacy.
In our study, 93 participants (both genders, 18-86 years of age) were recruited from community-dwelling individuals. Participants were informed of the trial design during initial presentations, which included randomization to treatment or placebo for the initial 3 months (referred to herein as the “Placebo Phase,” or Period 1, in accord with Zhang et al.), after which code would be revealed and all participants would receive treatment for the remainder of the study (Delayed-start Phase/Period 2). As suggested by Zhang et al., the knowledge that all participants would ultimately receive treatment greatly increased participation. At baseline and subsequent visits, participants completed the Trail-making test (parts A and B), which detects neuromuscular coordination difficulties and the ability to follow simple instructions (part A) and executive function (part B) [6-8]. Scores on A were subtracted from B to isolate effects on executive function. Group performance was statistically compared using an unpaired 1-tailed Student’s t test. In addition, each participant’s baseline score was subtracted from scores at subsequent intervals, and paired t distributions were calculated for each test interval vs. baseline, as well as for the treatment group versus delayed-start group (defined as “delta values” according to Zhang et al.).
The treatment group statistically improved during the placebo phase (i.e., the first 3 months; (p<0.04 versus baseline), while the delayed-start group did not (p<0.24; Fig. 1A); these groups differed statistically at this time (Δ1; p<0.04; Fig. 1A). At the 3-month visit, each participant was informed of prior group assignment. Placebo participants were informed of their switch to treatment (“delayed-start”; Fig. 1A), and after 3 months improved to a level statistically identical to that attained by the treatment group after their first 3 months of treatment (Δ2, p<0.39). The delayed-start group had therefore achieved a level of efficacy equivalent to that of the treatment group within the same respective treatment period.
Additional facets of our study support the RSD. Following the Delayed-start Phase, we withdrew treatment from all participants. After a 3 month “Washout” (Period 3), both groups had declined to levels statistically identical to baseline (p<0.20 and 0.47, respectively) and to each other (Δ3; Fig. 1A). Following resumption of treatment (Resume Phase/Period 4), both groups displayed statistically identical improvement (Δ4; Fig. 1A). These latter findings further indicate that the treatment and delayed-start groups had achieved and maintained statistical identity following the Delayed-start Period.
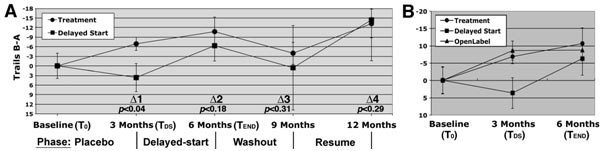
Zhang et al. point out that if the treatment has no DM effect and only provides symptomatic relief, then the delayed-start group will catch up to the treatment group. By contrast, should the treatment exert DM, the delayed-start group will likely not catch up, and will track differently from the treatment group throughout the remainder of the study. Since we utilized a healthy population, our findings are not intended to address DM but rather are directed entirely to symptomatic effects. The finding that our delayed-start group did indeed catch up to the treatment group, and moreover maintained statistical identity with it through further manipulations (washout and retreatment periods), support the hypothesis of Zhang et al. and, in doing so, provides evidence that RSD has the potential to reveal any DM effect over simple symptomatic effects.
Zhang et al. also address the important concern that code breaking and switching of prior placebo participants to a treatment group, as well as informing the prior treatment group of their status, may induce irregularities in results of an RSD. In this regard, a separate “open-label” cohort (all of which assigned to a treatment group and were aware of it) demonstrated improvement (p<0.04) that was identical to the first 3 months during which the treatment and delayed-start groups received the formulation (Fig. 1B). These findings indicate that the effect of codebreak is minimal and does not invalidate the use of RSD.
This demonstration supports the hypothesis of Zhang et al. that RSD has the capacity to distinguish DM versus symptomatic effects. Coupled with the potential for increased participation, and at least a reduction in the ethical concerns of functionally denying treatment to individuals at risk by maintaining protracted randomization, RSD represents a useful approach for testing novel interventions for AD.
Thomas B. Shea and Ruth Remington
University of Massachusetts • Lowell, Lowell MA 01854
References
[1] Speigel R., Berres M, Miserez AR, Monsch AU (2011) For debate: Substituting placebo controls in long-term Alzheimer’s prevention trials.Alzheimers Res Ther 3, 9-11.
[2] Zhang RY, Leon AC, Chuang-Stein C, Romano SJ (2011) A new proposal for randomized start design to investigate disease-modifying therapies for Alzheimer’s disease. Clin Trials 8, 5-14.
[3] Chan A, Lepore A, Kotoya E, Zemianek J, Remington R and Shea TB (2010) Efficacy of a vitamin/nutriceutical formulation on cognitive speed and recall in adults with no known or suspected dementia. J Nutr Health Aging 14, 224-230.
[4] Chan A, Paskavitz J, Remington R, Shea TB (2008) Efficacy of a vitamin/nutriceutical formulation for early-stage Alzheimer’s disease: A one-year open-label pilot study with a 16-month extension. Am J Alz Dis Other Dementias 23, 571-585
[5] Remington R, Chan A, Shea TB (2009) Efficacy of a vitamin/nutriceutical formulation for moderate to late-stage Alzheimer’s disease: A placebo-controlled pilot study. Am J Alz Dis Other Dementias 24, 27-33.
[6] Arbuthnott K and Frank J (2000) Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol 22, 518-528.
[7] Crowe SF (1998) The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the Trail Making Test. J Clin Psychol 54, 585-591.
[8] Tombaugh TN (2004) Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 19, 203-214.






