25 September 2022
New studies strengthen the case for links between the two diseases and demonstrate importance of good oral health
Preston, UK – Researchers at the School of Dentistry, University of Central Lancashire (UCLan) were the first to report the link between gum disease and Alzheimer’s disease. Now two new studies from the same research group at the School of Dentistry demonstrate that progress is being made in making much stronger connections between gum disease in the mouth and deteriorating brain function.
The studies are incredibly timely, as September is the World Alzheimer's month and today (21 September) is World Alzheimer's day. Marking this important awareness day, the two new studies – the first published in the Journal of Alzheimer’s Disease and the second in the Journal of Alzheimer’s Disease Reports – give a better understanding of the defining Alzheimer’s disease lesions on the brain: technically known as amyloid-beta plaques and neurofibrillary tangles.
Alzheimer's disease is the most common type of dementia, progressively causing deterioration of memory, thinking skills and the ability to communicate. The exact cause of this disease is not yet fully understood, which means it’s a difficult disease to prevent and treat.
It has been previously shown that the Porphyromonas gingivalis bacterium which destroys gum tissue - and the enzyme which it produces, known as gingipains - are specifically linked to Alzheimer’s disease, after both were discovered in the brain tissue of those suffering from the disease. These new studies go a step further in exploring how gum disease and its bacterial proteins can potentially contribute to the formation of lesions on the brain.
The first study, to be published in the Journal of Alzheimer’s Disease, shows that nerve cells in the brain contain a type of protein called tau. When tau meets the gingipains enzyme, the tau released from the nerve cell. Once freed, tau physically changes, in the form of coils and non-coiling filaments. These filaments of tau then re-attach to the nerve cell and become incorporated into the lesion known as neurofibrillary tangles. These ultimately kill the nerve cells.
What this means is that once a nerve cell dies and the free tau protein leaks into the brain, the tau may attach itself to healthy neighbouring nerve cells, repeating the process and leading to further damage to the brain as the disease spreads.
The second study, published in the Journal of Alzheimer’s Disease Reports, looks at the way the gingipains enzyme, released by the bacterium, can contribute to the formation of amyloid-beta plaques – another of the lesions, alongside the tangles, which form on the brains of those suffering from Alzheimer’s.
These studies are small steps in the fight against Alzheimer’s, but the results are significant in understanding the role of gingipains and how fundamental they are to lesion formation. It is hoped that studies such as these will help with developing future treatments.
Shalini Kanagasingam, Specialist Endodontist and Senior Clinical Lecturer at UCLan, who led the study, (supervised by Dr Sim K. Singhrao) said: “What this kind of research proves is the importance of our oral health. Look out for early signs of gum disease such as bleeding gums when brushing or more advanced signs like movement or drifting of the teeth. Don’t delay or skip your dental check-ups. Your dentist will be able to help advise you on how to effectively remove plaque and tartar from your teeth, which harbour the bacterium that we have identified as a risk factor for Alzheimer’s. These studies highlight the key message that a healthy mouth is important for maintaining a healthy body and mind.”
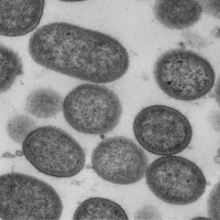
Photo caption: Porphyromonas gingivalis, which secretes the deadly enzyme gingipains (credit: UCLan)
###
NOTES TO EDITORS
Full study: "Antimicrobial, Polarizing Light, and Paired Helical Filament Properties of Fragmented Tau Peptides of Selected Putative Gingipains" by S. Kanagasingam, C. von Ruhland, R. Welbury, and S.K. Singhrao (DOI: 10.3233/JAD-220486), published early online in the Journal of Alzheimer’s Disease, in advance of Volume 90, Issue 1. It is available online at: content.iospress.com/articles/journal-of-alzheimers-disease/jad220486.
Full open access study: "Porphyromonas gingivalis Conditioned Medium Induces Amyloidogenic Processing of the Amyloid-β Protein Precursor upon in vitro Infection of SH-SY5Y Cells" by S. Kanagasingam, C. von Ruhland, R. Welbury, S.S. Chukkapalli, S.K. Singhrao (DOI: 10.3233/ADR-220029), published in the Journal of Alzheimer’s Disease Reports in Volume 5, Issue 1. It is available online at: content.iospress.com/articles/journal-of-alzheimers-disease-reports/adr220029.
Contact
For more information contact, Hannah Watkins Media Relations Manager, University of Central Lancashire (+44 7935173992 or hrwatkins@uclan.ac.uk).
About the University of Central Lancashire
The University of Central Lancashire (UCLan) in Preston has an established research reputation with world-leading or internationally excellent work taking place within the areas of Business, Health, Humanities and Science. As a truly global institution with an established campus in Cyprus, UCLan’s student body includes 120 nationalities and its partnership network extends to 125 countries. In 2021 the Center for World University Rankings placed UCLan in the top 7 percent of all worldwide universities. uclan.ac.uk
About the Journal of Alzheimer’s Disease
Now in its 25th year of publication, the Journal of Alzheimer’s Disease (JAD) is an international multidisciplinary journal to facilitate progress in understanding the etiology, pathogenesis, epidemiology, genetics, behavior, treatment, and psychology of Alzheimer’s disease. The journal publishes research reports, reviews, short communications, book reviews, and letters-to-the-editor. Groundbreaking research that has appeared in the journal includes novel therapeutic targets, mechanisms of disease, and clinical trial outcomes. JAD has a Journal Impact Factor of 4.160 according to Journal Citation Reports (Clarivate, 2022). The journal is published by IOS Press. j-alz.com







